Plumbing the Depths of Liquid Crystals

When Christine McGinn '16 requested a research post in the lab of Morris L. Clothier Professor of Physics Peter Collings, she expected more grunt work than glory.
By the end of the summer, however, she had enough theoretical and through-the-microscope knowledge of liquid crystals to be the first author of a publication in Physical Review.

Collings recently co-authored a paper on liquid crystals with a Penn research team that made the front cover of Proceedings of the National Academy of Sciences.
"It completely blew my expectations out of the water," says McGinn, an engineering/physics major and women's lacrosse defender from Sewickley, Pa. "I couldn't have imagined being handed an important responsibility as a rising sophomore, let alone to be published.
"That's one of the amazing aspects about working with [Collings], though," she adds. "He does good work that people in the scientific community care about."
Liquid crystals are soft matter that exist in a state between conventional liquids and solid crystals and enable the displays of most computer monitors, TVs, and smartphones. McGinn spent last summer adding chiral material to a new type of water-based liquid crystal and measuring the degree to which it would twist.
"They kind of looked like blue squiggly lines," she says, "interconnecting and going left or right when twisting through the cell. It was like putting together a weird puzzle."
McGinn would share her findings with Collings, who would help her determine the next steps. Incorporating the technique of aligning the liquid crystals on glass developed the previous summer by Laura Laderman '15, a physics major from San Francisco, Calif., Collings wrote the Physical Review paper with the Swatties, and two other researchers with whom he had collaborated last year in Germany, as co-authors.
"I don't often bring students [such as McGinn and Laderman] who have only completed one year into my lab," says Collings, "but they were wonderful."
The effort built upon Collings' more than 15 years of work with Swarthmore students on water-based liquid crystals, examining their complexities and controllability. He recently brought that knowledge to a research group at the University of Pennsylvania, where he holds a faculty appointment, resulting in another high-profile publication.
A postdoctoral fellow and a graduate student whom Collings helps supervise there led the effort, which made it to the cover of the Proceedings of the National Academy of Sciences. The group examined the same type of liquid crystal that dissolves in water, rather than the oily liquid crystals that are found in electronic displays. Penn News describes their process:
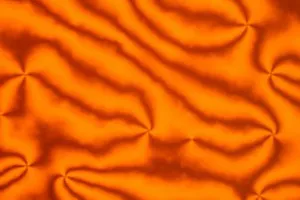
Liquid crystals viewed in a polarizing microscope.
The researchers placed these liquid crystals into water droplets, which in turn were placed in oil, producing an emulsion. At high enough concentrations within the droplets, the liquid crystals exhibit a twisting pattern visible under an optical microscope. At even higher concentrations, however, the liquid crystals display an even more unusual behavior: their constituent molecules stack to form columns that organize into crystal-like structures and transform the normally spherical water droplets into faceted fluid gemstones.
Seeing the surface of the droplets evolve to look like gemstones amazed the researchers. But when they stopped to think about it, the phenomenon made sense, Collings says, since the material's hexagonal order can create the illusion of an actual crystal.
"That doesn't take away from the initial surprise and, you might even say, awe," he says, "but it quickly becomes, 'If I had been smarter, I would have thought of that.'"
The key takeaway is that these types of liquid crystals may have biomedical applications, "which hasn't been possible until now," Collings says. That's because they're mostly water, with a complex internal structure that supports life processes.
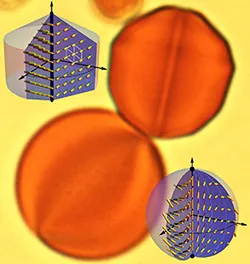
Lyotropic chromonic liquid crystals (LCLC) droplets in columnar (above) and nematic (below) phases.
Realizing those biomedical applications won't happen quickly or easily, but by continuing to investigate the interfaces between soft matter systems, Collings says researchers will unlock their potential.
"Until now, most of the work has been preliminary," he says. "We didn't know how to control or orient the liquid crystals. But we're getting better at that."
The high-profile publications and steps forward in researchers' understanding of liquid crystals come at a fitting time for Collings, who begins a three-year phased retirement schedule in September. The forward momentum is likely to continue, however, given the impact he had on students and colleagues at Swarthmore and beyond.
"[Collings] is just a fantastic teacher and mentor," says McGinn, adding that he helped her with negotiating her double major and exploring summer employment. "I can't say enough about how great it was to collaborate with him."
Also remaining is the research aspect of the physics and astronomy program, which encourages students to delve deeply into a niche as they study the fundamental areas within a field. This is a common element of the science programs at Swarthmore, as evidenced by the 50 or so students - rising sophomores among them - conducting a wide variety of research on campus each summer.
"We think an important part of their education is helping them figure out what they'd like to do later on," says Collings. "There's nothing better than scientific inquiry to help them answer that question."