Scientific Instrument Makes Leap from Lab to Historical Significance
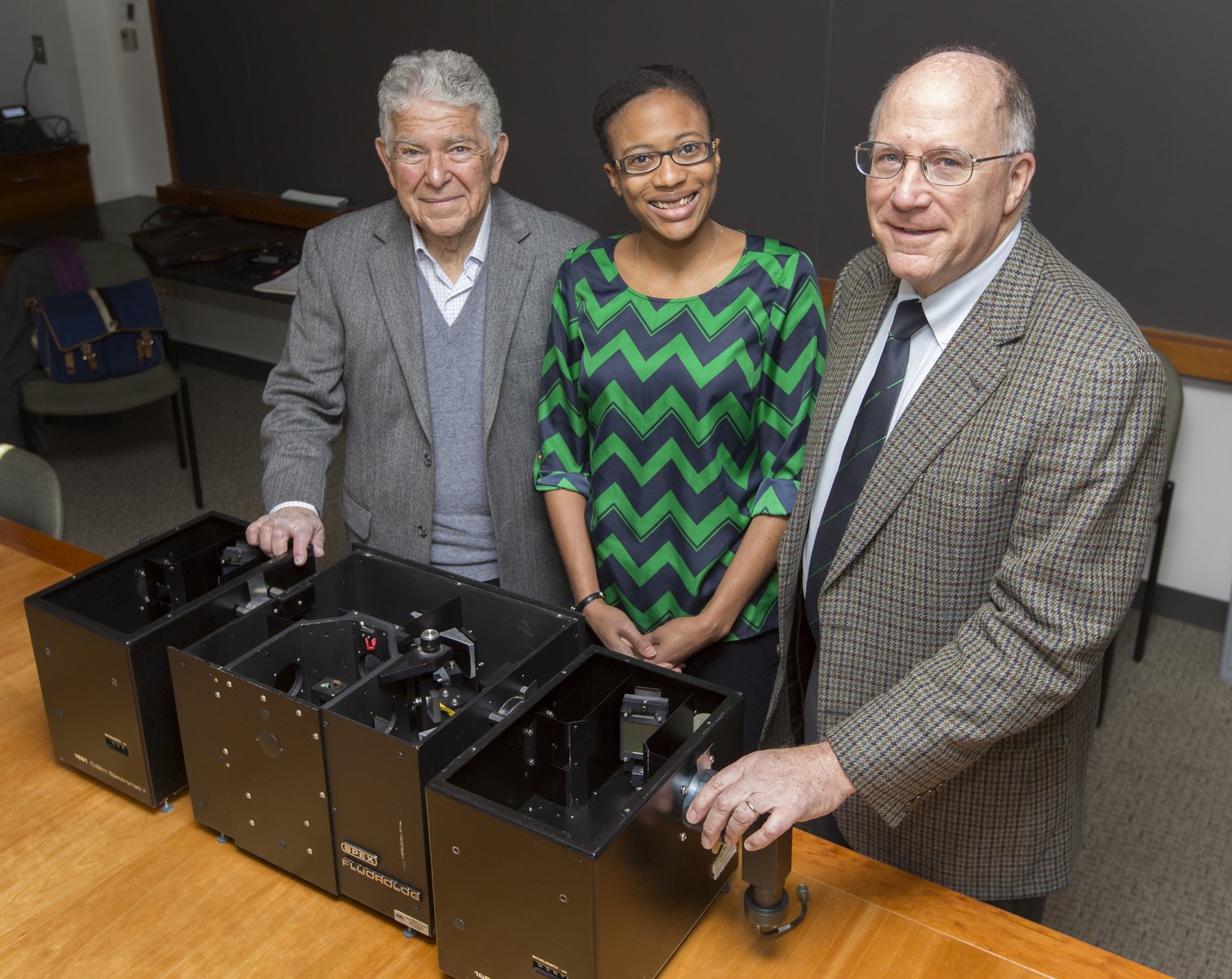
Bob Pasternack, Stephanie Lampkins of the Chemical Heritage Foundation, and Peter Collings with the historically significant SPEX Fluorolog Spectrometer.
Bob Pasternack and Peter Collings didn’t set out to break new ground.
The chemist and the physicist, chance collaborators, just wanted a better sense of an organic compound’s usefulness in chemotherapy. In the process, they pioneered the now widely used technique of resonance light scattering.
But it’s the way they went about it, stretching the limits of existing equipment, that’s the story.
That story will continue to be told at the Chemical Heritage Foundation (CHF), which came to Swarthmore last month to commemorate Pasternack's and Collings' discovery and claim for its collection the SPEX Fluorolog Spectrometer that the duo used to make the fortuitous discovery in 1992.
“We found the story behind how they applied the instrument to make this discovery intriguing,” says Stephanie Lampkin, CHF's museum collections manager. “We’re excited and grateful to add this to our collection.”
That story began when Pasternack, then the Edmund Allen Professor of Chemistry and Biochemistry at the College, found that water-soluble porphyrins could form what appeared to be a microarray on DNA. He wanted to examine that more closely and identified light scattering as his best option.
“So, I turned to the expert,” says Pasternack, now emeritus professor and senior research scholar.
“We collaborated on a problem where the typical physics way of doing things or the typical chemistry way of doing things wouldn’t yield the whole answer,” adds Collings, Morris L. Clothier Professor of Physics. “It was about using ideas from both fields and pushing things as far as we could to get what we needed.”
Their early experiments, with fixed wavelength lasers in Collings’ research lab, yielded some conclusions but left many more questions unanswered. They knew that monitoring the light scattering over a range of wavelengths would help but weren’t sure how to do that.
“Then it occurred to me that we already had an instrument that could, with a little modification, be used for light scattering,” says Pasternack.
Right away, with the fluorometer set up in an unusual configuration, they saw dramatic results. The only question was whether these results were unique. The pair made some calls and reviewed the literature, Collings says. “And it turned out that we had discovered something brand new.”
After publishing their findings, Pasternack and Collings incorporated resonance light scattering into their general research efforts at Swarthmore. Dozens of students used this technique and co-authored papers with the professors over the following decade.
Pasternack and Collings also traveled the world to showcase their discovery. One of the reasons it became so popular among scientists is because it uses equipment that is already sitting in their labs. Even the most cash-strapped researcher could now explore the world of supramolecular chemistry.
“I’d go right into their labs to demonstrate it,” Pasternack says. “The response was just remarkable, and there have now been more than 1,000 papers published using these methods.”
The pair also collaborated with chemists from Haverford and Goucher Colleges on a project funded by the National Science Foundation, before phasing the fluorometer out in 2010.
Thanks to the Chemical Heritage Foundation, though, the instrument leaps from the shelf to historical significance.
“It has great research potential for our current and future fellows and outside scholars,” says Lampkin, “and future exhibition potential.”
Asked now whether he could have foreseen the instrument’s legacy, Collings laughs.
“I don’t think our thinking was anywhere close to that direction,” he says. “We were just trying to figure out how to perform some measurements and tried something that turned out to be pretty useful to folks.”
Pasternack concurs, but he also recalls Pasteur: “Chance favors the prepared mind.”
“In this case,” he says, smiling, “two prepared minds.”