The developing lung presents a good model system in which to study organ formation because it is composed of only two types of cells. The lining of the bronchioles and alveoli are made of epithelium, surrounded by a loosely-packed mesenchyme that functions to produce and secrete signaling molecules. These proteins bind to receptors in the cell membrane of the epithelial cells and signal them to divide and differentiate.
The epithelium of the lung is initially formed as a simple tube, which branches to form the left and right sides of the organ. Subsequently, several rounds of proliferation and branching produce a "tree" of bronchioles, and the tips expand to form the alveoli. Finally, the cells differentiate. The epithelium of the alveoli becomes very thin to allow gas exchange and cells of the bronchioles start to produce surfactant, a protein that coats the inside of the lungs and allows the alveoli to expand with air without being damaged. If the lung epithelium is incompletely developed at birth, with inadequate production of surfactant, this results in neonatal respiratory distress syndrome, a major cause of infant mortality.
Branching of the epithelium of the mammalian lung requires signals from the adjacent mesenchyme. The best candidate 'branching signal' is Fibroblast Growth Factor 10 (FGF10). FGF10 is expressed in patches in the mesenchyme adjacent to the site of a future branch, and it induces chemotaxis and proliferation of isolated lung epithelium. FGF10 also induces the delayed expression of Bone Morphogenetic Protein4 (BMP4). It has been suggested that directed chemotaxis towards FGF10 is responsible for both new bud formation and bud outgrowth, and that induction of BMP4 subsequently limits the outgrowth of the new lung buds....

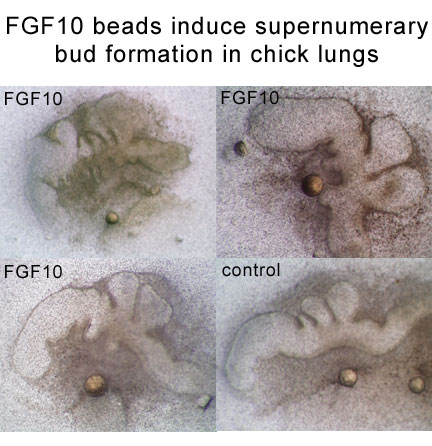
To investigate the role of FGF signaling during lung branching morphogenesis, we examined the growth of avian and murine lungs in organ culture.To determine if a localized source of FGF10 was sufficient to stimulate new bud formation, we exposed the primary bronchus of embryonic chick lungs to a bead soaked in purified FGF10. We found that a single bead could induce the formation of a series of regularly spaced new buds. This suggests that exposure to FGF10 is sufficient to induce bud formation, but that factors in addition to FGF10 concentration are involved in the spacing of new buds.
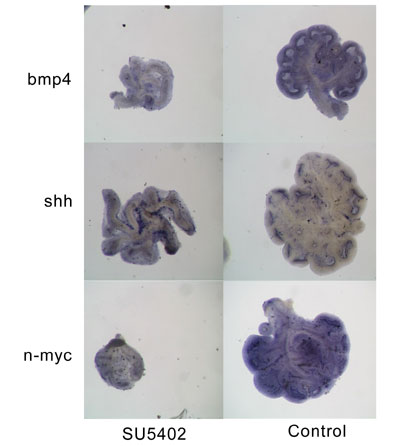
To determine if FGF signaling is required for both bud formation and outgrowth, we cultured embryonic mouse lungs in the presence of an inhibitor of both type I and type II FGF receptors (SU5402, SUGEN, Inc). As predicted, this resulted in the abrogation of new bud formation. However, the existing branches continued to grow and became abnormally elongated. This suggests that the early response to FGF10 activates the mechanisms necessary for bud elongation, which continues until halted by later responses.

The expression of BMP4 is lost or reduced in the existing buds of lungs treated with SU5402. This supports the view that an increase in bmp4 expression is a late response to FGF10 signaling and is necessary to limit lung bud outgrowth. The expression of both shh and n-myc is also reduced. These genes are normally expressed at higher levels in the epithelial tips and both have been linked to cell proliferation. This suggests that the continuing lung bud outgrowth may be primarily driven by cell shape changes that continue even when FGF10 signaling is blocked.