|
|
|
|
||
|
|
|
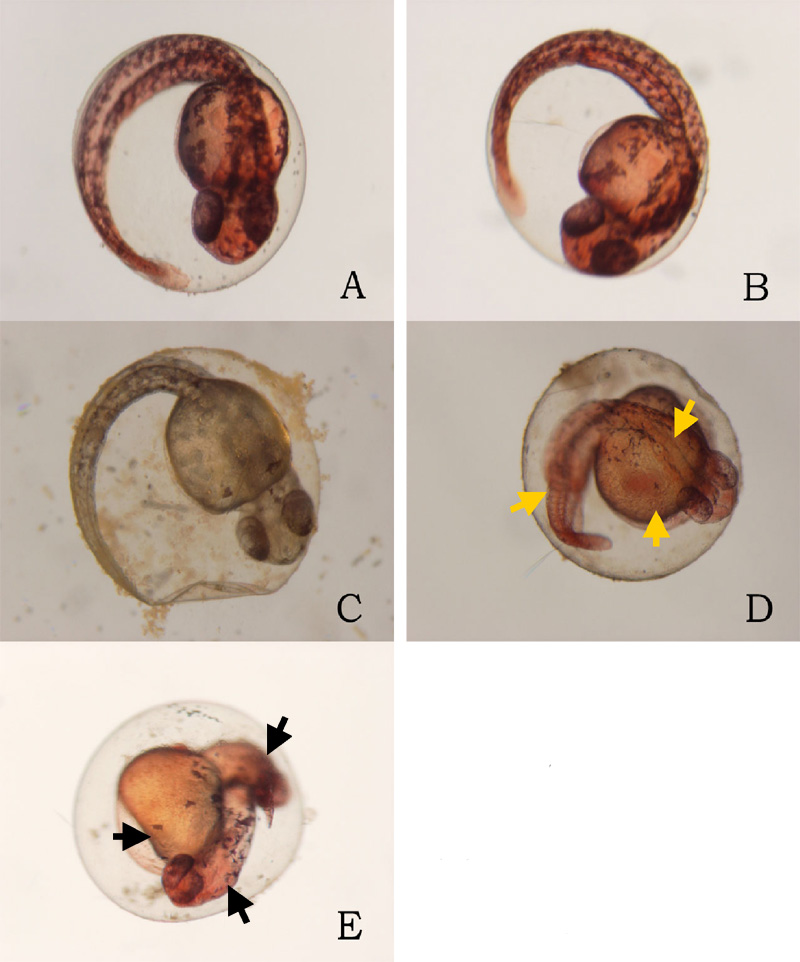
24 hours after the exposure of ethanol, cyclopia was observed among the embryos cultured at 3% ethanol (Figure 1, Table 1). No cyclopia was observed in embryos at 1% or 2% ethanol solutions. For both concentrations, eyes developed normally; the vesicles were darkly pigmented and the eyes were normally spaced (Figures 1B, 1C). At 3%, 1 cyclopic embryo was observed. The rest of the surviving embryos showed no signs of cyclopia. The cyclopic embryo’s eyes were fused and were deficient in pigmentation (Figure 1D).
In addition to cyclopia, embryos exposed to 3% ethanol
showed varying degrees of impaired development of posterior
structures, more specifically of the trunk and tail, 24
hours after their exposure to ethanol (Figure
3). Again, no deformities were observed in the posterior
regions of those at 1% and 2% ethanol solutions (Figures 3B,
3C). 2 embryos, 1 of whom was the aforementioned cyclops,
cultured in 3% ethanol solution displayed malformation of
the trunk and tail (Figures 3D, 3E). The non-cyclopic embryo
(eyes were not fused but had shortened distance between
them) with posterior deformities had enlarged allantois with
a shortened tail (Figure 3D). Moreover, much of the
pigmentations normally observed along the dorsal side of
untreated embryos were absent (Figure 3D). The cyclopic
embryo displayed similar symptoms with increased severity
(Figure 3E). At the 48 hour stage, there were no posterior deformities observed among embryos cultured at 1% and 2% ethanol solution (Figure 4, Table 1). All surviving embryos grown at 1% and 2% solution that hatched displayed normal swimming movements. At 3%, the cyclopic embryos with deformed posterior structures continued to develop with the aforementioned deformities which, at the 48 hour stage, ultimately rendered the embryos immobile. The non-cyclopic embryo with defective trunk and tail died. The cyclopic embryo failed to hatch, and when artificially pulled out of the shell, did not show normal swimming motions. In fact, the embryo was completely immobile due to the severity of its trunk and tail deformites.
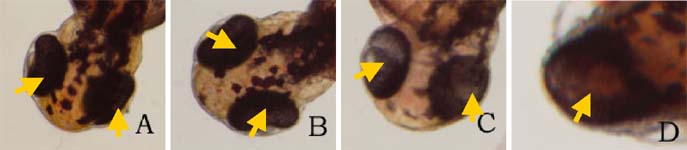
 Click on the picture to view the enlarged version Figure 1. Ethanol treatment causes
cyclopia at 3%. Zebrafish embryos treated at the dome/30%
epiboly stage with 1%,2%, and 3% ethanol- Zebrafish Embryo
Medium (ZEM) solution for 3 h and cultured in ZEM for 24 h
in the absence of ethanol. No cyclopia was observed in 1%
and 2%. 1 cyclopic embryo was observed out of 10 at 3%. (A)
Untreated control embryo. (B) 1% ethanol treated embryo. (C)
2% ethanol treated embryo. (D) 3% ethanol with cyclopia. Eye
vesicles are fused along the midline of the head. Arrows
indicate eyes. Click on the picture for enlarged
version.
 Click
on the picture to view the enlarged version Click
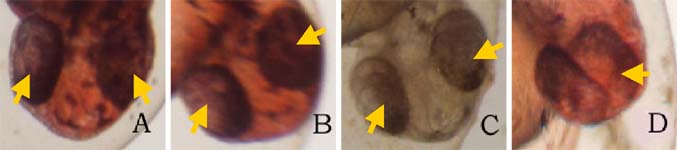
on the picture to view the enlarged versionFigure. 2. Ethanol treatment causes cyclopia at 3%. Embryos treated at the dome/30% epiboly stage with increasing concentration of ethanol for 3 h and cultured for 48 h in Zebrafish Embryo Medium in the absence of ethanol. Cyclopia observed at 3% only. (A) Untreated control embryo. (B) 1% ethanol treated embryo. (C) 2% ethanol treated embryo. (D) 3% ethanol with cyclopia. Eye vesicles are fused along the midline of the head. Embryos are shown in frontal views, dorsal up. Arrows indicate eyes. Click on the picture for enlarged version. FIG. 3. Ethanol treatment impairs trunk and tail development at 3%. Embryos were treated from the dome/30% epiboly stage with increasing concentration of ethanol for 3 h and then cultured for 24 hours in the Zebrafish Embryo Medium in the absence of ethanol. (A) Untreated control embryo. (B) 1% ethanol treated embryo. (C) 2% ethanol treated embryo. (D) Non-cyclopic embryo generated by 3% ethanol treatment showing mild deformities of the trunk and tail. (E) Cyclopic embryo generated by 3% ethanol treatment showing severe trunk and tail deformities. Arrows indicate defective trunk and tail. FIG. 4. Ethanol treatment impairs trunk and tail development at 3%. Embryos were treated from the dome/30% epiboly stage with increasing concentration of ethanol for 3 h and then cultured for 48 hours in the Zebrafish Embryo Medium in the absence of ethanol. (A) Untreated control embryo. (B) 1% ethanol treated embryo. (C) 2% ethanol treated embryo. (D) Cyclopic embryo generated by 3% ethanol treatment showing severe trunk and tail deformities. Arrows indicate trunk and tail deformities.
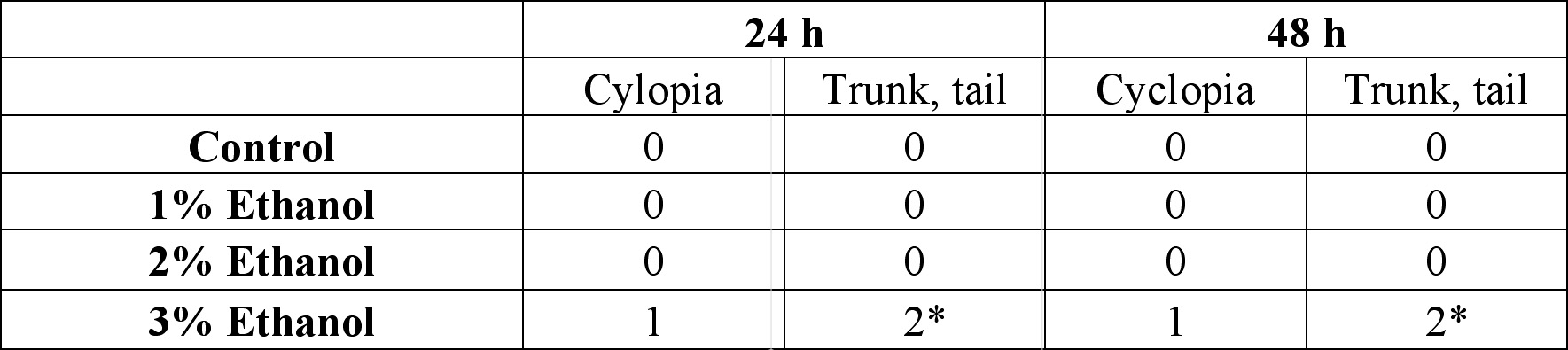
Table 1. Developmental abnormalities induced by ethanol exposure. Embryos were treated from the dome/30% epiboly stage with 1%, 2%, and 3% ethanol- Zebrafish Embryo Medium (ZEM) solution for 3 h and cultured in the ZEM for a total of 48h in the absence of ethanol. 10 embryos were subjected to each concentration. Cultures were observed twice: 24 h after the treatment and 48 h after the treatment. Dead embryos with deformities were not counted.
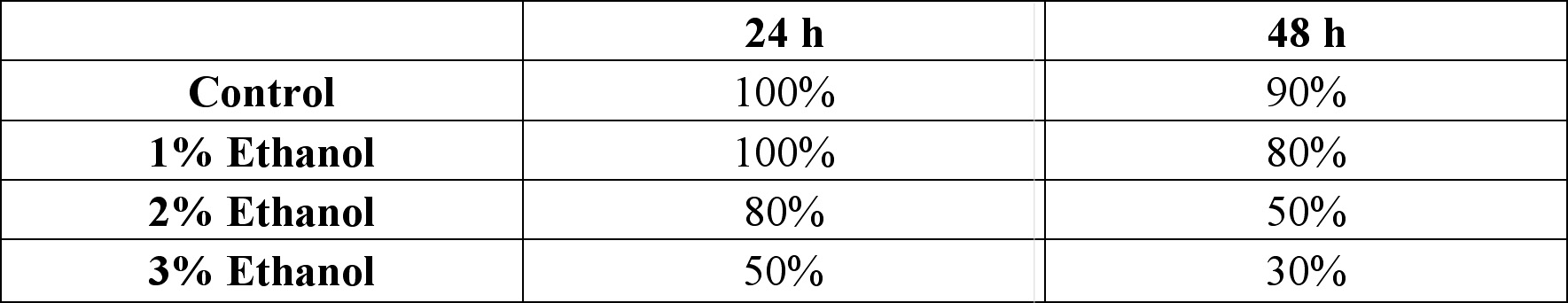
*One of the 2 also had cyclopia, the same embryo denoted by 1 in the 24 cyclopia column. Table 2. Viability of zebrafish embryos exposed to different concentration of ethanol concentrations at two 24 h intervals. Embryos were treated from the dome/30% epiboly stage with with 1%, 2%, and 3% ethanol- Zebrafish Embryo Medium (ZEM) solution for 3 h and cultured in the ZEM for a total of 48h in the absence of ethanol. 10 embryos were exposed to each concentration. Cultures were checked twice: 24 h after the treatment and 48 h after the treatment.
|
Last Modified: 10 May 2004
[Lab Protocols | Students | Cebra-Thomas | Course | Links ]