|
|
|
|
|
|
|
|
||
|
|
|
Procedure: 1. Dechorionate 24 hour zebrafish embryos in the pharyngula stage with 2 fine forceps. 2. Fix approximately of the embryos with 4%, and the other half in 1% paraformaldehyde in PBS for one hour. 3. Wash embryos 3x in 5 ml PBS to remove fixative 4. Incubate embryos in PBS with goat serum and 0.2% saponin 5a. Add the primary
antibody, 5b. Add the primary
antibody, 6. Wash embryos with several changes of 5 ml PBS to remove antibody 7. Incubate with the secondary
antibody, 8. Wash with several changes of PBS 9. Mount with depression slides and observe stained embryos using the fluorescent microscope. 10. Look for individual brightly stained cells in the tail region.
Figure 1. Dechorionated Zebrafish embryo at approximately 24 hours 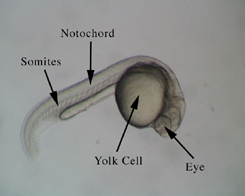 |
|
Last Modified: 2 August 2001
[Lab
Protocols
| Students
| Cebra-Thomas
| Course
| Links
]